The CA19-9 IRMA system provides a direct in vitro quantitative determination of the cancer associated antigen CA19-9 in human serum in the range of 0-240 U/mL.
Each kit contains material sufficient for 100 or 50 assay tubes, permitting the construction of one standard curve and the assay of 42 (RK-199CT) or 17 (RK-199CT50) unknowns in duplicate.
Introduction
CA19-9 is a tumour associated mucin-type high molecular weight glycoprotein antigen circulating in the blood and can be found in tissues. This test is useful in monitoring patients with confirmed pancreatic cancer whose CA19-9 levels are above the cut off at the time of the diagnosis. Patients negative for the Lewis blood group antigen have no CA19-9 in their blood even in the presence of malignant disease.
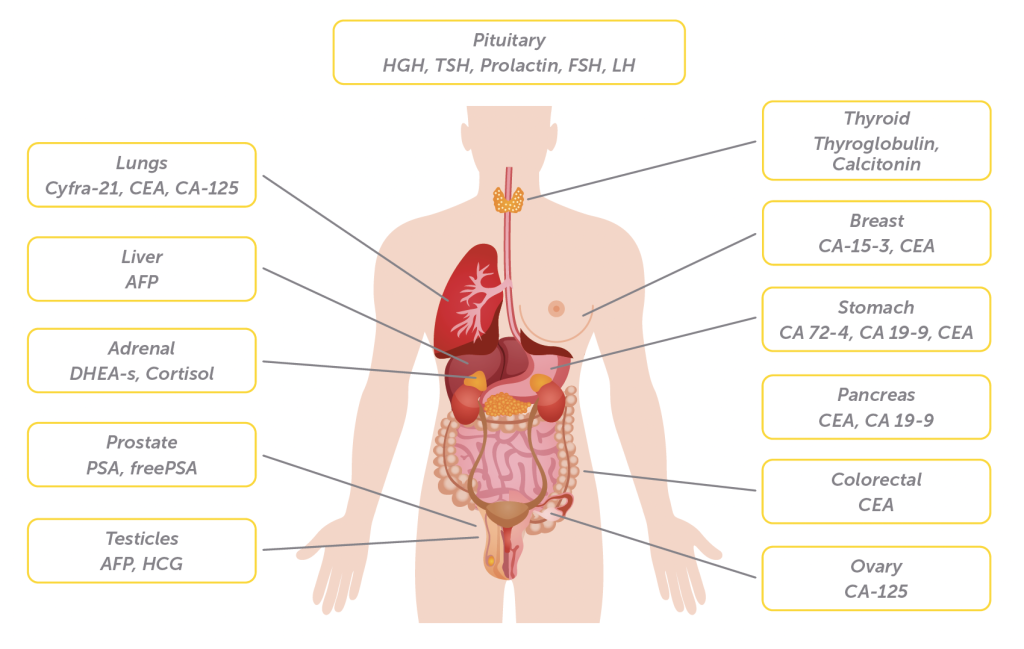
One of the primary advantages of using RIA for tumour marker analysis is its exceptional sensitivity. RIA can detect tumour markers in extremely low concentrations. Each tumour marker has its own specificity and sensitivity, making it suitable for particular cancer types or stages. By utilizing a panel of tumour markers, clinicians can obtain a comprehensive profile of the patient’s disease status.
Please, click here for our complete tumour marker portfolio.